Structure of the Atom
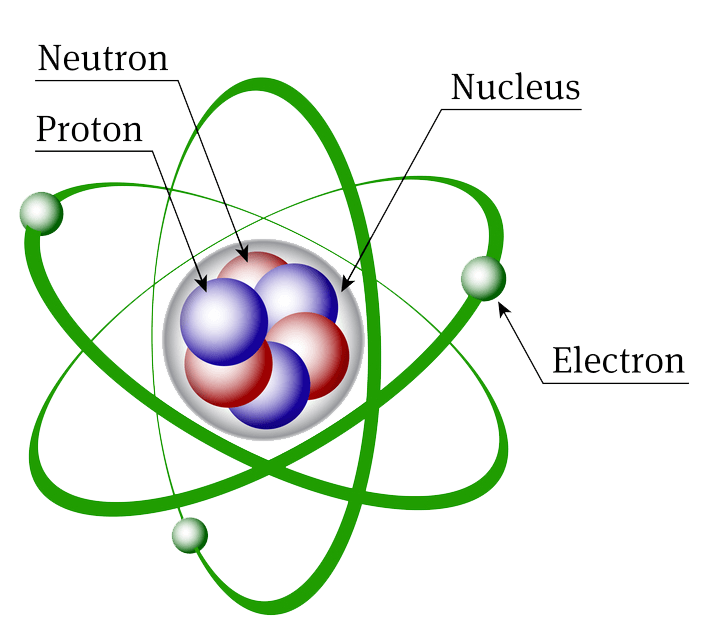
The atoms of which every element of matter is composed have a nucleus at the center and electrons whirling about this nucleus that can be visualized as planets circling around a sun, though it is impossible to locate them precisely within the atom. The nuclei of atoms are composed of protons, which have a positive electrical charge, and neutrons, which are electrically neutral. Electrons are electrically negative and have a charge equal in magnitude to that of a proton.
The number of electrons in an atom is normally equal to the number of protons in the nucleus. As a result, atoms of elements are normally electrically neutral. The mass of an atom lies almost entirely in its nucleus since protons and neutrons are far heavier than electrons.
Free neutrons are unstable particles which decay naturally into a proton and electron, with a half-life of about 12 minutes.
neutron ===> proton + electron + a neutrino
However, it is remarkable that neutrons, when they exist together with protons in the nucleus of atoms, are stable. Protons are about 1,836 times heavier than electrons, and neutrons are about 1,838 times heavier than electrons. The energy balance in the decay of a neutron is achieved by the anti-neutrino, a neutral particle that carries off surplus energy as the neutron decays.
The nominal mass of an atom of an element is measured by the sum of the protons and neutrons in it. This integer is called the mass number. The nominal mass of an atom is not affected by the number of electrons, which are very light. Hence the nominal mass, based on the mass number, approximates the actual atomic mass. The number of protons in the nucleus, which determines the chemical properties of an element, is called the atomic number. Elements are arranged in ascending order of atomic number in an arrangement called the periodic table. The term derives from the tendency to periodicity of chemical properties deriving from arrangements of electrons in atoms.
The number of electrons in an atom is normally equal to the number of protons in the nucleus. As a result, atoms of elements are normally electrically neutral. The mass of an atom lies almost entirely in its nucleus since protons and neutrons are far heavier than electrons.
Free neutrons are unstable particles which decay naturally into a proton and electron, with a half-life of about 12 minutes.
neutron ===> proton + electron + a neutrino
However, it is remarkable that neutrons, when they exist together with protons in the nucleus of atoms, are stable. Protons are about 1,836 times heavier than electrons, and neutrons are about 1,838 times heavier than electrons. The energy balance in the decay of a neutron is achieved by the anti-neutrino, a neutral particle that carries off surplus energy as the neutron decays.
The nominal mass of an atom of an element is measured by the sum of the protons and neutrons in it. This integer is called the mass number. The nominal mass of an atom is not affected by the number of electrons, which are very light. Hence the nominal mass, based on the mass number, approximates the actual atomic mass. The number of protons in the nucleus, which determines the chemical properties of an element, is called the atomic number. Elements are arranged in ascending order of atomic number in an arrangement called the periodic table. The term derives from the tendency to periodicity of chemical properties deriving from arrangements of electrons in atoms.